Building a resilient medicine supply chain to combat tuberculosis in the Kyrgyz Republic
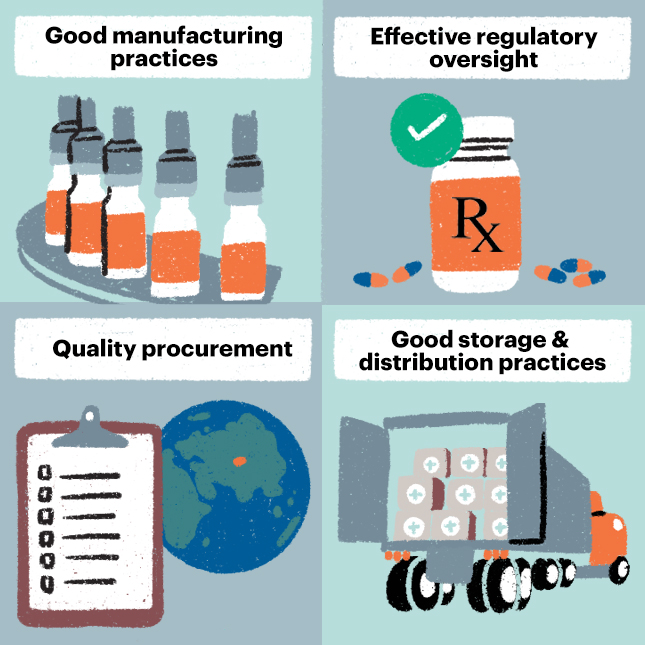
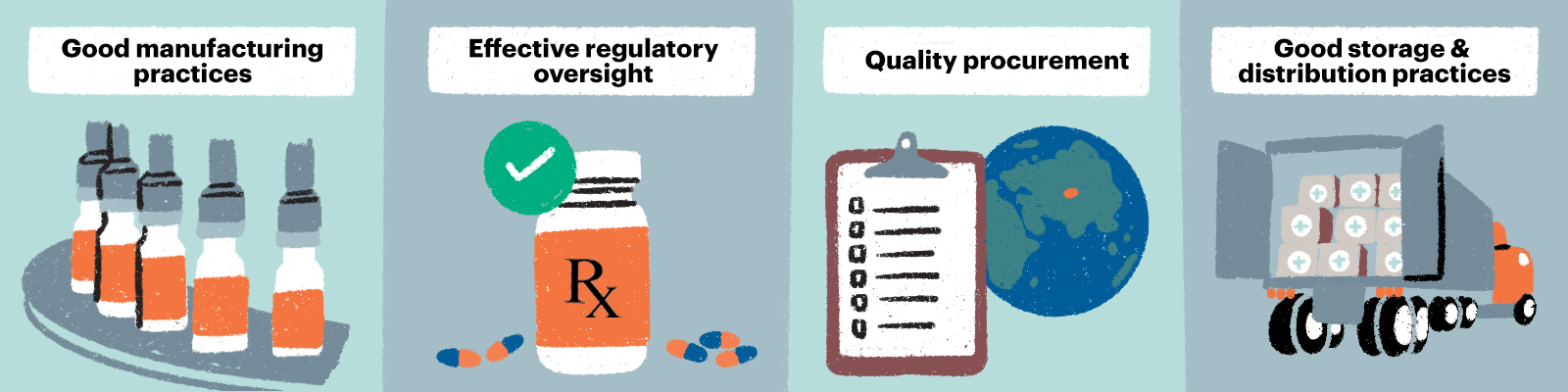
Ensuring quality of TB medicines across the supply chain saves lives
Preventing, treating, and curing patients with drug-resistant tuberculosis (DR-TB) is a major public health challenge in the Kyrgyz Republic and worldwide. In fact, the Kyrgyz Republic has one of the highest rates of multidrug-resistant tuberculosis (MDR-TB) in the world.
In 2020, nearly 7,000 people in the Kyrgyz Republic developed TB, 1,000 of whom were diagnosed with DR-TB.
According to the World Health Organization (WHO), 29 percent of new TB cases in the Kyrgyz Republic are DR-TB, compared with 3.3 percent worldwide.