What is a dietary supplements monograph?
Monographs set the standard for each dietary ingredient or formulation and are used to ensure quality dietary supplements are available to the public. Monographs set forth the article’s name, definition, specifications, and other requirements related to packaging, storage, and labeling. Specifications include tests, procedures, and acceptance criteria that help ensure identity, strength, quality and purity. These tests and analytical procedures have been validated following the directions of the General Chapter <1225> Validation of Pharmacopeial Procedures.
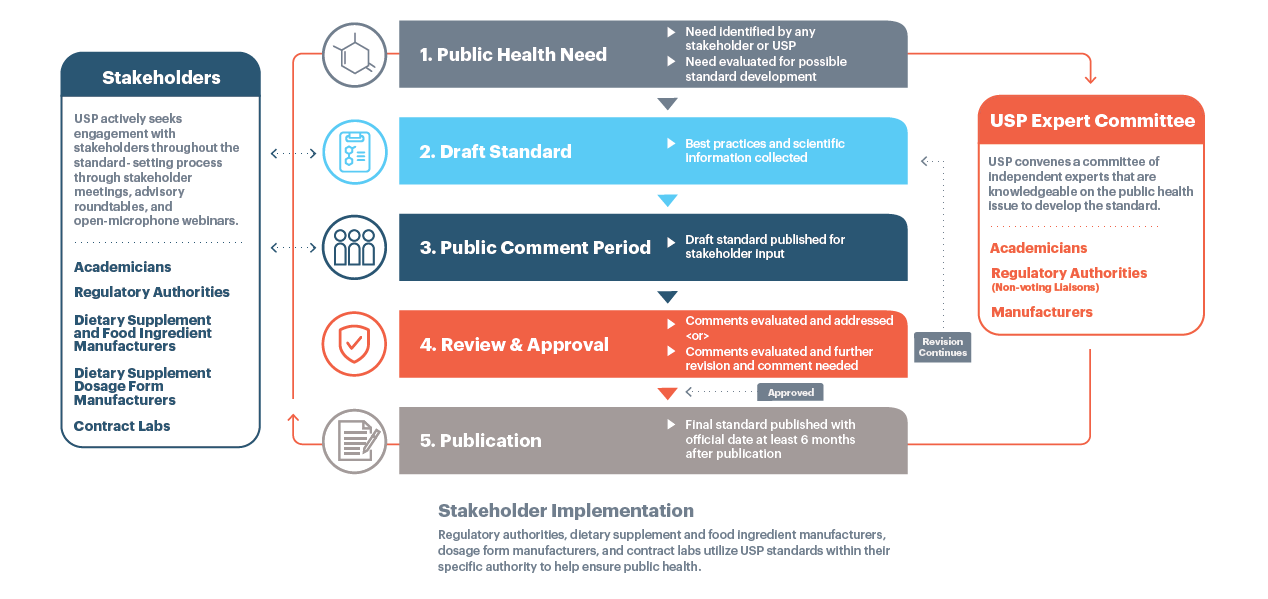
How are monographs developed?
Monograph development is a multi-step process. Technical data contributed by manufacturers and ingredient or product developers may serve as the foundation for a draft proposal that is reviewed by an Expert Committee that is ultimately responsible for its approval. Draft proposals are available for 90 days in the Pharmacopeial Forum (PF) to receive comments stakeholders.
Why contribute to USP’s monograph development process?
Working with USP to develop a new monograph brings an independent review of ingredient specifications, manufacturing processes, testing methods and potential candidates for reference standard materials. USP donors will receive the Reference Standard Candidate Evaluation Package, a summary of the data collected by USP on the candidate reference material, upon release into inventory.
By setting official USP standards, you are helping to protect your product category, elevate the quality of your ingredients, and reduce the flow of adulteration at a global level. USP standards are legally recognized in the regulatory frameworks of many countries.
Questions about becoming a USP donor? Reach out to the Dietary Supplements & Herbal Medicines Science Team.


